THERMODYNAMICS LAWS
Laws of thermodynamics
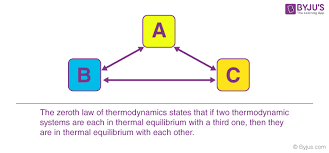
Zeroth Law
The zeroth law of thermodynamics states: If two systems are each in thermal equilibrium with a third, they are also in thermal equilibrium with each other.
This statement implies that thermal equilibrium is an equivalence relation on the set of thermodynamic systems under consideration. Systems are said to be in equilibrium if the small, random exchanges between them (e.g. Brownian motion) do not lead to a net change in energy. This law is tacitly assumed in every measurement of temperature. Thus, if one seeks to decide whether two bodies are at the same temperature, it is not necessary to bring them into contact and measure any changes of their observable properties in time. The law provides an empirical definition of temperature, and justification for the construction of practical thermometers.
The zeroth law was not initially recognized as a separate law of thermodynamics, as its basis in thermodynamical equilibrium was implied in the other laws. The first, second, and third laws had been explicitly stated already, and found common acceptance in the physics community before the importance of the zeroth law for the definition of temperature was realized. As it was impractical to renumber the other laws, it was named the zeroth law.
First Law
The first law of thermodynamics states: In a process without transfer of matter, the change in internal energy, , of a thermodynamic system is equal to the energy gained as heat, , less the thermodynamic work, , done by the system on its surroundings
For processes that include transfer of matter, a further statement is needed: With due account of the respective fiducial reference states of the systems, when two systems, which may be of different chemical compositions, initially separated only by an impermeable wall, and otherwise isolated, are combined into a new system by the thermodynamic operation of removal of the wall, then
- ,
where U0 denotes the internal energy of the combined system, and U1 and U2 denote the internal energies of the respective separated systems.
Adapted for thermodynamics, this law is an expression of the principle of conservation of energy, which states that energy can be transformed (changed from one form to another), but cannot be created or destroyed.[26]
Internal energy is a principal property of the thermodynamic state, while heat and work are modes of energy transfer by which a process may change this state. A change of internal energy of a system may be achieved by any combination of heat added or removed and work performed on or by the system. As a function of state, the internal energy does not depend on the manner, or on the path through intermediate steps, by which the system arrived at its state.
Second Law
A traditional version of the second law of thermodynamics states: Heat does not spontaneously flow from a colder body to a hotter.
The second law refers to a system of matter and radiation, initially with in homogeneity in temperature, pressure, chemical potential, and other intensive properties, that are due to internal 'constraints', or impermeable rigid walls, within it, or to externally imposed forces. The law observes that, when the system is isolated from the outside world and from those forces, there is a definite thermodynamic quantity, its entropy, that increases as the constraints are removed, eventually reaching a maximum value at thermodynamic equilibrium, when the inhomogeneities practically vanish. For systems that are initially far from thermodynamic equilibrium, though several have been proposed, there is known no general physical principle that determines the rates of approach to thermodynamic equilibrium, and thermodynamics does not deal with such rates. The many versions of the second law all express the irreversibility of such approach to thermodynamic equilibrium.
In macroscopic thermodynamics, the second law is a basic observation applicable to any actual thermodynamic process; in statistical thermodynamics, the second law is postulated to be a consequence of molecular chaos.
Third Law
This law of thermodynamics is a statistical law of nature regarding entropy and the impossibility of reaching absolute zero of temperature. This law provides an absolute reference point for the determination of entropy. The entropy determined relative to this point is the absolute entropy. Alternate definitions include "the entropy of all systems and of all states of a system is smallest at absolute zero," or equivalently "it is impossible to reach the absolute zero of temperature by any finite number of processes".
Absolute zero, at which all activity would stop if it were possible to achieve, is −273.15 °C (degrees Celsius), or −459.67 °F (degrees Fahrenheit), or 0 K (kelvin), or 0° R (degrees Rankine).









Comments
Post a Comment